How Many Electrons Can Fit in the 3rd Energy Level
The energy levels or shells correspond with the letters K L M N O P and Q respectively. Following the formula the third energy level can contain 18 electrons the fourth energy level can hold 32 electrons the fifth energy level can hold 50 electrons the sixth energy level can carry up to 72 electrons and the seventh energy level can contain 98 electrons.

How Many Electrons Can The Third Energy Level Hold At Level
Find step-by-step Biology solutions and your answer to the following textbook question.
. The maximum number of electrons in the 3s sublevel is two the maximum number of electrons in the 3p subsevel is six and the maximum number of electrons in the 3d sublevel is ten. How many subshells does the 3rd energy level have. How many electrons can fit in the third energy level.
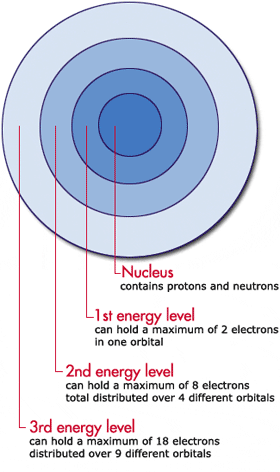
2 Get Other questions on the subject. A flask containing 450 ml of 050 m h2so4 was accidentally knocked to the floor. Therefore the second level can contain a maximum of eight electrons that is two in the s orbital and 6 in the three p orbitals.
When there are no excited electrons energy levels fill up innermost to outermost. Therefore there are 3 subshells in the. N 8 18 32 Question 14 3 pts Which atom has an electron configuration of Ar4s23d104p3.
View the answer now. So 2 6 10 8. How many electrons can fit in the 3rd energy levelshell.
In an investigation that uses the scientific method which step immediately follows making a hypothesis. How many electrons are in the third energy level. I did the Gizmos.
The answer is B. The maximum number of electrons on any given level can be determined by the formula two times. How many electrons can fit in the third energy level.
The Gizmo shows all of the first two energy levels but only part of the third energy level. How many electrons are in the second energy level. A is 2 B is 8 and C is 18.
How many electrons are in the third energy level. The second energy level has 6 electrons. How many electrons can an third energy level hold.
How many grams of nahco do you need to put on the spill to neutralize the acid according to the following. How many electrons can fit in the second energy level. O summarizing the results o asking a question o making observations designing an experiment mark this.
Each principal energy level above the second contains in addition to one s orbital and three p orbitals a set of five d orbitals called the d sublevel. The third energy level n3 contains the 3s 3p and 3d sublevels. Maximum of 18 electrons in the third energy level.
There is no third energy level of oxygen there is only two. The third energy level of an atom referred to as the M shell can hold a maximum of 18 electrons. The number of electrons that any particular element has in the third level depends on its location on the periodic table and can range from none to 18 electrons.
There are 2 electrons in excited states the ones in the third energy level. The energy levels are typically referred to by their shell number rather than their energy level. Following the formula the third energy level can contain 18 electrons the fourth energy level can hold 32 electrons the fifth energy level can hold 50 electrons.
This means that before the second shell can begin being filled it must have a full first shell. How many electrons can. Chemistry questions and answers.
Thus the first energy level holds 2 12 2 electrons while the second holds 2 22 8 electrons. 2 Show answers Another question on Chemistry. These can be visualised in the following Aufbau diagram.
O Arsenic O Vanadium O Phosphorus O Aluminum. How many electrons can fit in the first energy level. Chemistry 22062019 0740 sadcase85.
In electron configuration the third energy level is divided into the following subshells. Electrons are arranged in orbits called energy levels.
How Many Electrons Can The Third Energy Level Hold At Level
Interactives The Periodic Table It S Elementary For A Mad Scientist

No comments for "How Many Electrons Can Fit in the 3rd Energy Level"
Post a Comment